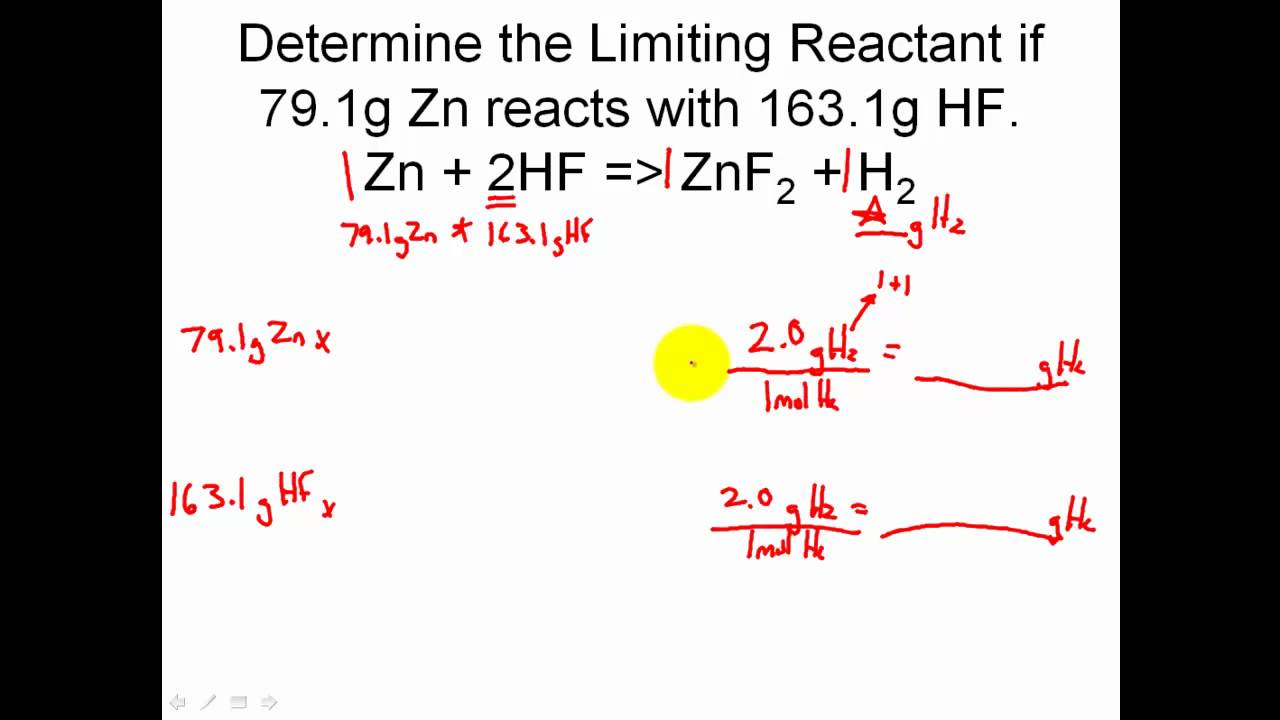
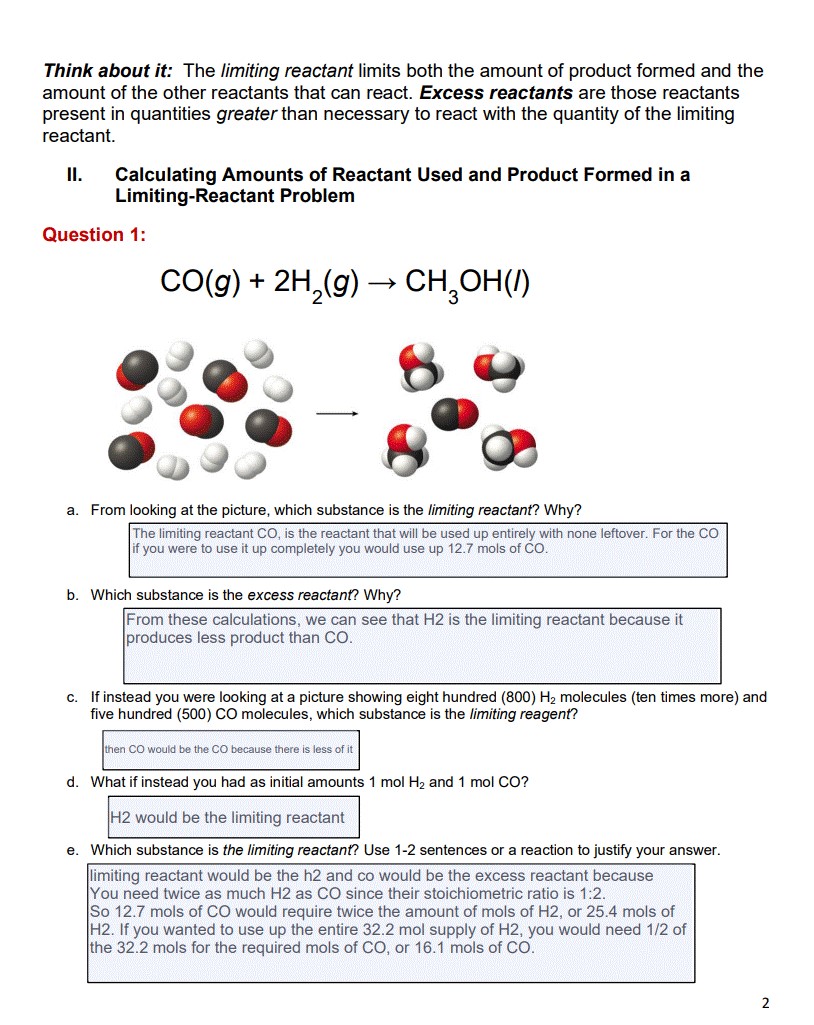
Limiting Reactant Khan Academy
Limiting Reactant Khan Academy - Limiting reactant example problem here’s a complex example problem with a limiting reactant worked out in detail. Learn about limiting reactants, reaction yields, and how to calculate them in chemical reactions. A) 3 atoms of carbon combine with 4 molecules of. Calculating the amount of product formed from a limiting reactant Determine limiting and excess reagent and the amount of unreacted excess reactant.
Learn about limiting reactants, reaction yields, and how to calculate them in chemical reactions. A) 3 atoms of carbon combine with 4 molecules of. Determine limiting and excess reagent and the amount of unreacted excess reactant. Limiting reactant example problem here’s a complex example problem with a limiting reactant worked out in detail. Calculating the amount of product formed from a limiting reactant
A) 3 atoms of carbon combine with 4 molecules of. Learn about limiting reactants, reaction yields, and how to calculate them in chemical reactions. Determine limiting and excess reagent and the amount of unreacted excess reactant. Limiting reactant example problem here’s a complex example problem with a limiting reactant worked out in detail. Calculating the amount of product formed from a limiting reactant
Limiting reactant (example problem) Chemistry Khan Academy Urdu
A) 3 atoms of carbon combine with 4 molecules of. Determine limiting and excess reagent and the amount of unreacted excess reactant. Limiting reactant example problem here’s a complex example problem with a limiting reactant worked out in detail. Learn about limiting reactants, reaction yields, and how to calculate them in chemical reactions. Calculating the amount of product formed from.
Limiting reactant example problem 1 Chemistry Khan Academy (With
Limiting reactant example problem here’s a complex example problem with a limiting reactant worked out in detail. Learn about limiting reactants, reaction yields, and how to calculate them in chemical reactions. A) 3 atoms of carbon combine with 4 molecules of. Determine limiting and excess reagent and the amount of unreacted excess reactant. Calculating the amount of product formed from.
Limiting reactant example problem 1 Chemistry Khan Academy YouTube
Determine limiting and excess reagent and the amount of unreacted excess reactant. A) 3 atoms of carbon combine with 4 molecules of. Limiting reactant example problem here’s a complex example problem with a limiting reactant worked out in detail. Learn about limiting reactants, reaction yields, and how to calculate them in chemical reactions. Calculating the amount of product formed from.
Limiting Reactants. ppt download
Limiting reactant example problem here’s a complex example problem with a limiting reactant worked out in detail. Determine limiting and excess reagent and the amount of unreacted excess reactant. Calculating the amount of product formed from a limiting reactant Learn about limiting reactants, reaction yields, and how to calculate them in chemical reactions. A) 3 atoms of carbon combine with.
Limiting Reactant Example Worksheet
Learn about limiting reactants, reaction yields, and how to calculate them in chemical reactions. Calculating the amount of product formed from a limiting reactant Limiting reactant example problem here’s a complex example problem with a limiting reactant worked out in detail. Determine limiting and excess reagent and the amount of unreacted excess reactant. A) 3 atoms of carbon combine with.
how to solve limiting reactant
Learn about limiting reactants, reaction yields, and how to calculate them in chemical reactions. Limiting reactant example problem here’s a complex example problem with a limiting reactant worked out in detail. Determine limiting and excess reagent and the amount of unreacted excess reactant. A) 3 atoms of carbon combine with 4 molecules of. Calculating the amount of product formed from.
SOLUTION Limiting reactant detailed topic Studypool
Learn about limiting reactants, reaction yields, and how to calculate them in chemical reactions. Determine limiting and excess reagent and the amount of unreacted excess reactant. Calculating the amount of product formed from a limiting reactant A) 3 atoms of carbon combine with 4 molecules of. Limiting reactant example problem here’s a complex example problem with a limiting reactant worked.
Limiting Reactant Worksheet Answers Englishworksheet.my.id
A) 3 atoms of carbon combine with 4 molecules of. Learn about limiting reactants, reaction yields, and how to calculate them in chemical reactions. Determine limiting and excess reagent and the amount of unreacted excess reactant. Limiting reactant example problem here’s a complex example problem with a limiting reactant worked out in detail. Calculating the amount of product formed from.
Solved Think about it The limiting reactant limits both the
Calculating the amount of product formed from a limiting reactant Learn about limiting reactants, reaction yields, and how to calculate them in chemical reactions. Determine limiting and excess reagent and the amount of unreacted excess reactant. A) 3 atoms of carbon combine with 4 molecules of. Limiting reactant example problem here’s a complex example problem with a limiting reactant worked.
Limiting Reactants GCSE Lesson (SC9c CC9c) Teaching Resources
Determine limiting and excess reagent and the amount of unreacted excess reactant. Calculating the amount of product formed from a limiting reactant Limiting reactant example problem here’s a complex example problem with a limiting reactant worked out in detail. A) 3 atoms of carbon combine with 4 molecules of. Learn about limiting reactants, reaction yields, and how to calculate them.
Limiting Reactant Example Problem Here’s A Complex Example Problem With A Limiting Reactant Worked Out In Detail.
Learn about limiting reactants, reaction yields, and how to calculate them in chemical reactions. Calculating the amount of product formed from a limiting reactant A) 3 atoms of carbon combine with 4 molecules of. Determine limiting and excess reagent and the amount of unreacted excess reactant.