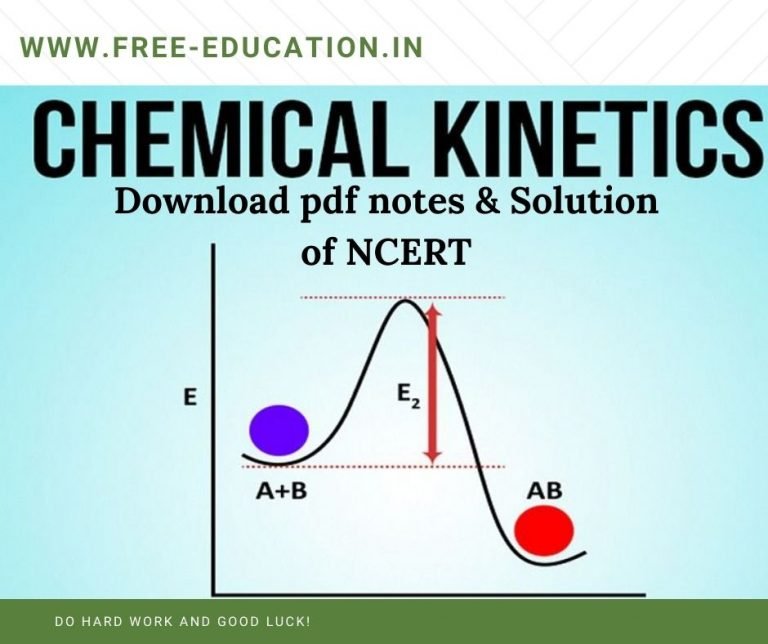
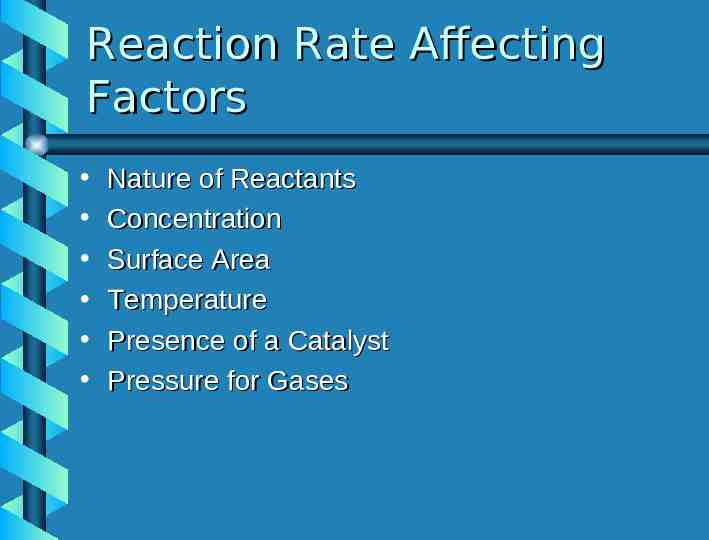
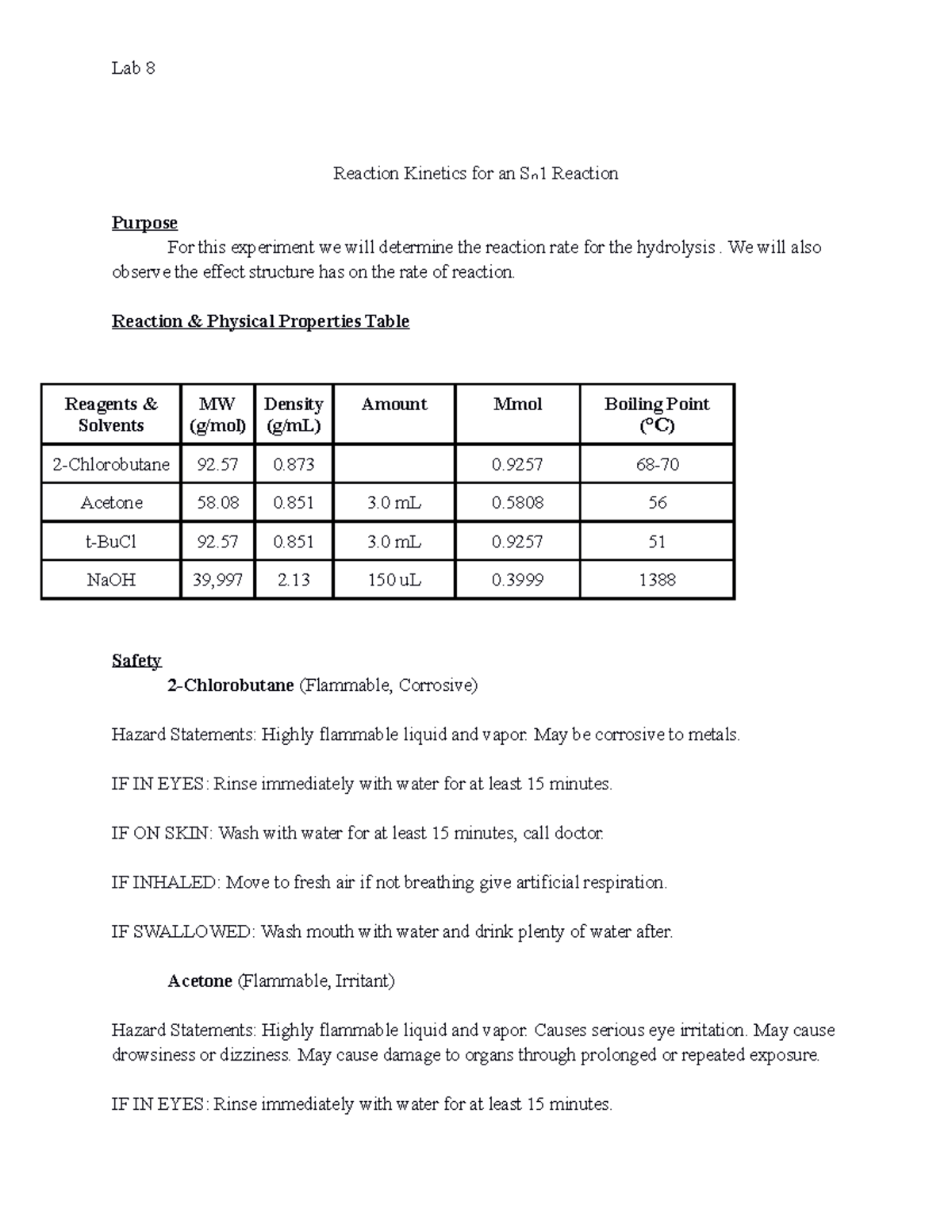
Reaction Kinetics Khan Academy
Reaction Kinetics Khan Academy - Chemical kinetics average rate of reaction. Many reaction mechanisms contain one step that is much slower than the others; Check out the next lesson and practice what you’re learning: And these two molecules are isomers of each other. The rate of a chemical reaction is defined as the rate of change in concentration of a reactant or product divided by its coefficient.
And these two molecules are isomers of each other. The rate of a chemical reaction is defined as the rate of change in concentration of a reactant or product divided by its coefficient. Chemical kinetics average rate of reaction. Check out the next lesson and practice what you’re learning: Many reaction mechanisms contain one step that is much slower than the others;
The rate of a chemical reaction is defined as the rate of change in concentration of a reactant or product divided by its coefficient. And these two molecules are isomers of each other. Chemical kinetics average rate of reaction. Many reaction mechanisms contain one step that is much slower than the others; Check out the next lesson and practice what you’re learning:
Reaction and Equilibrium How compounds react with each other
Chemical kinetics average rate of reaction. Many reaction mechanisms contain one step that is much slower than the others; The rate of a chemical reaction is defined as the rate of change in concentration of a reactant or product divided by its coefficient. And these two molecules are isomers of each other. Check out the next lesson and practice what.
CBSE Class 12th Chemistry Chemical Notes Wisdom TechSavvy
Chemical kinetics average rate of reaction. And these two molecules are isomers of each other. The rate of a chemical reaction is defined as the rate of change in concentration of a reactant or product divided by its coefficient. Check out the next lesson and practice what you’re learning: Many reaction mechanisms contain one step that is much slower than.
Reaction and Equilibrium How compounds react with each other
Check out the next lesson and practice what you’re learning: And these two molecules are isomers of each other. Many reaction mechanisms contain one step that is much slower than the others; The rate of a chemical reaction is defined as the rate of change in concentration of a reactant or product divided by its coefficient. Chemical kinetics average rate.
Group 3Reaction essentialsA20 1 st Quarter S. 2022
And these two molecules are isomers of each other. Chemical kinetics average rate of reaction. Many reaction mechanisms contain one step that is much slower than the others; The rate of a chemical reaction is defined as the rate of change in concentration of a reactant or product divided by its coefficient. Check out the next lesson and practice what.
Reaction and Equilibrium How compounds react with each other
Check out the next lesson and practice what you’re learning: Many reaction mechanisms contain one step that is much slower than the others; The rate of a chemical reaction is defined as the rate of change in concentration of a reactant or product divided by its coefficient. And these two molecules are isomers of each other. Chemical kinetics average rate.
Reaction Notes for Class11+12 Umair Khan Academy
The rate of a chemical reaction is defined as the rate of change in concentration of a reactant or product divided by its coefficient. Check out the next lesson and practice what you’re learning: Many reaction mechanisms contain one step that is much slower than the others; Chemical kinetics average rate of reaction. And these two molecules are isomers of.
Reaction and Equilibrium How compounds react with each other
Many reaction mechanisms contain one step that is much slower than the others; And these two molecules are isomers of each other. The rate of a chemical reaction is defined as the rate of change in concentration of a reactant or product divided by its coefficient. Check out the next lesson and practice what you’re learning: Chemical kinetics average rate.
Reaction AACB1113 Physical Chemistry TAR UC Thinkswap
Many reaction mechanisms contain one step that is much slower than the others; Check out the next lesson and practice what you’re learning: Chemical kinetics average rate of reaction. And these two molecules are isomers of each other. The rate of a chemical reaction is defined as the rate of change in concentration of a reactant or product divided by.
Lab 8 Reaction for an Sn1 Reaction Reaction for an
The rate of a chemical reaction is defined as the rate of change in concentration of a reactant or product divided by its coefficient. Chemical kinetics average rate of reaction. And these two molecules are isomers of each other. Check out the next lesson and practice what you’re learning: Many reaction mechanisms contain one step that is much slower than.
Reaction and Equilibrium How compounds react with each other
Many reaction mechanisms contain one step that is much slower than the others; Chemical kinetics average rate of reaction. Check out the next lesson and practice what you’re learning: The rate of a chemical reaction is defined as the rate of change in concentration of a reactant or product divided by its coefficient. And these two molecules are isomers of.
Many Reaction Mechanisms Contain One Step That Is Much Slower Than The Others;
Chemical kinetics average rate of reaction. And these two molecules are isomers of each other. The rate of a chemical reaction is defined as the rate of change in concentration of a reactant or product divided by its coefficient. Check out the next lesson and practice what you’re learning: